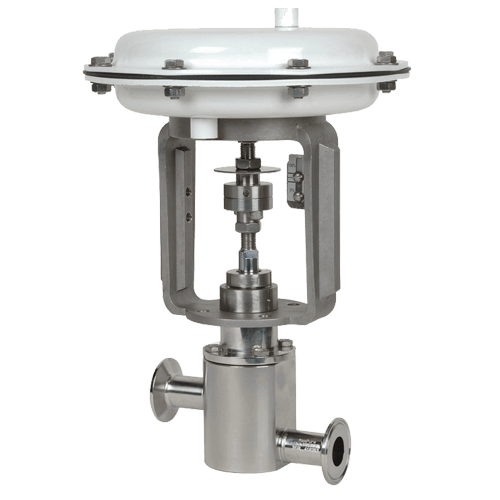
Chromatography Buffer Elution Flow Control
Mark 978JD Series ⟶
Medium to high flow, Jorlon diaphragm
Mark 978M Series ⟶
Manual control, low to high flow, Jorlon diaphragm
Mark 978INLINE Series ⟶
Medium to High Flow, Jorlon Diaphragm
Your List
Email List
Get A Quote
Accuracy and consistency are fundamental requirements for biopharmaceutical manufacturing equipment. Among the many processes that ensure product quality, chromatography buffer elution flow control plays a crucial role. This application manages the precise delivery of buffer solutions during the chromatography process, allowing researchers and manufacturers to achieve reproducible, high-purity results.
By regulating how buffer solutions are introduced and how elution occurs, chromatography buffer elution flow control ensures the stability of the separation process. This precision is essential for applications like chromatography purification, protein separation, and large-scale pharmaceutical production.
Why Buffer Elution Flow Control Matters in Chromatography
During chromatography, molecules are separated based on their interactions with a stationary phase and a mobile buffer phase. For this separation to be efficient, the flow of buffer must be carefully managed. Poor flow control leads to inconsistent elution patterns, loss of resolution, and lower product yields.
With proper Chromatography Buffer Elution Flow Control, manufacturers gain:
- Reproducibility: Ensures consistent buffer flow across multiple batches.
- Purity: Optimizes separation, reducing contamination risks.
- Scalability: Enables seamless transition from lab-scale chromatography purification to full-scale biopharmaceutical manufacturing equipment.
- Efficiency: Minimizes waste of costly buffer solutions.
This makes buffer flow control not just a technical requirement but a foundation for reliable, high-quality drug development and production.
Applications of Chromatography Buffer Elution Flow Control
Chromatography buffer elution flow control is widely used in biopharma and life sciences industries. Key applications include:
- Protein Purification: Achieving precise elution profiles for monoclonal antibodies, enzymes, and therapeutic proteins.
- Biopharmaceutical Manufacturing: Supporting large-scale buffer delivery systems that meet strict quality and compliance standards.
- Chromatography Purification Research: Helping scientists maintain reproducible results in R&D environments.
- Process Scale-Up: Enabling smooth transfer of methods from laboratory development to industrial production.
In every stage, buffer flow regulation ensures that separation remains accurate, controlled, and compliant with global regulatory expectations.
Steriflow Valve Solutions for Buffer Elution Flow Control
At Steriflow, we understand the challenges of maintaining precise buffer management in critical applications. To support this, we provide advanced systems specifically designed for Chromatography Buffer Elution Flow Control.
Our recommended series include:
- Mark 978JD Series: Engineered for high accuracy and stability in controlling buffer flow during chromatography purification.
- Mark 978M Series: Designed for consistent buffer delivery in both laboratory and production-scale environments.
- Mark 978INLINE Series: Provides streamlined, in-line flow control for optimized buffer elution in biopharmaceutical processes.
Each series has been developed with the unique requirements of biopharmaceutical manufacturing equipment in mind, ensuring reliability, precision, and long-term performance.
Benefits of Choosing Steriflow Valve
Implementing chromatography buffer elution flow control with Steriflow systems offers multiple advantages:
- High Accuracy: Maintains precise buffer flow rates across varying pressures.
- Scalable Performance: Solutions adaptable for research labs, pilot plants, and commercial production.
- Compliance-Ready: Designed to meet regulatory standards for pharma and biotech.
- Efficiency and Cost Savings: Reduces buffer waste and increases production reliability.
With decades of expertise, Steriflow delivers trusted solutions that help leading pharma and biotech companies achieve success in their chromatography applications.
Driving Innovation in Biopharmaceutical Manufacturing
As the demand for advanced therapies continues to grow, so does the need for dependable and precise process control. Chromatography buffer elution flow control is no longer just an option-it’s a necessity for ensuring safe, consistent, and cost-effective production.
At Steriflow, we are committed to supporting this progress. Our technologies not only provide effective buffer management but also align with the evolving needs of chromatography purification and biopharmaceutical manufacturing equipment.
Bringing Precision to Every Chromatography Process
The success of chromatography depends heavily on buffer management. By implementing chromatography buffer elution flow Control, manufacturers can achieve higher product quality, improved process consistency, and greater efficiency.
Steriflow’s Mark 978JD, Mark 978M, and Mark 978INLINE Series are engineered to deliver precisely that-robust, scalable, and compliant solutions for modern chromatography needs.
Whether in research, scale-up, or full production, Steriflow stands as a trusted partner for ensuring reliable buffer elution control and advancing the future of biopharmaceutical manufacturing equipment.